Background
All-trans retinoic acid (ATRA) has been used with great success in acute promyelocytic leukemia (APL) cases with PML::RARA fusion gene (FG). There are still PML::RARA negative cases manifest as APL. So far, 19 RARA fusion partner genes have been reported, with all reported as 5′ partners ( X::RARA). Intriguingly, a significant proportion of X::RARA cases were resistant to ATRA, and mechanisms are yet to be elucidated. Here we report the finding of a novel type of trinary fusion and the orchestrating of multiple molecular events that confer leukemogenesis and ATRA resistance in X::RARA-APL.
Methods
A total of 23 cases (Fig. 1A) with RNA-seq and WGS data from 10 centers were enrolled. We used Arriba for routine FG calling and manually investigated the nonconventional fusion sequence. RT-PCR, Sanger sequencing, and optical genome mapping (OGM) were used to validate the FGs and to determine whether the RARA 5′ and 3′ fusion events were located on the same cistron. The responsiveness of the fusion proteins to ATRA was evaluated using an optimized UAS/GAL4 reporter system.
Results
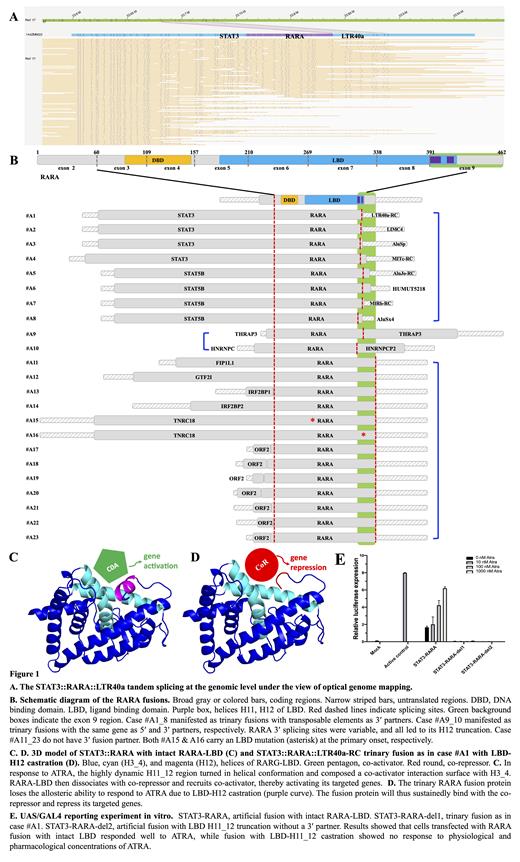
Among the cases enrolled, there were 17 males and 6 females, aged 3-66 (median 32) years. Morphological and immunophenotype analyses all showed features of APL. We first identified the STAT3::RARA fusion event in an ATRA-resistant case (the index case, #A1). Moreover, we noticed another fusion event of truncated RARA exon 9 and a 3′ partner sequence that did not map to any annotated protein-coding genes. Further analysis determined it was the RC sequence of the transposable element (TE) LTR40a with the annotated loci at 17p11.2. Both OGM (at the genome level) and RT-PCR (Fig. 1A) confirmed that the two RARA fusion events were in the same cistron. Thus, the fusion transcript being STAT3::RARA::LTR40a-RC spliced in tandem, but not separate transcripts of STAT3::RARA and RARA::LTR40a-RC. We named this novel form of tandem splicingtrinary fusion. We, therefore, recruit X::RARA-APL cases to investigate the recurrence of RARA 3′ fusion.
We identified 9 RARA 5′ partner genes in 23 cases (Fig. 1B), with TTMV -ORF2, STAT5B, STAT3, IRF2BP1, THRAP3, and TNRC18 in multiple cases. Ten (43.5%) cases also harbor RARA 3′ fusion partners. The partner of case #A1_8 were TEs, and the partner of case #A9_10 was the same gene as their 5′ one. Thirteen cases did not harbor 3′ fusions. Notably, both TNRC18::RARA cases (#15_16) carry mutations on their RARA ligand binding domain (LBD) at the primary onset. Gene expression analysis confirmed the aberrant activation of the involved TEs. All TEs involved in the RARA 3' fusions confer a poly_A signal sequence, which was essential for a mRNA. In-depth analysis indicated that TEs participate in forming RARA-FGs through a transposition mechanism rather than a translocation mechanism.
The molecular events addition to RARA 5′ splicing showed a 5′ partner associated pattern: all of the 4 STAT3::RARA and 4 STAT5B::RARA cases harbor TEs as their 3′ partner, both TNRC18::RARA cases carry LBD mutation, while all 7 TTMV -ORF2::RARA cases did not harbor 3′ splicing or LBD mutation. Remarkably, although the RARA 3' splicing sites varied, they all resulted in partial or complete exon 9 truncation.
RARA exon 9 encodes helix 11_12 (H11_12) of its LBD, which is pivotal in allosteric response to ATRA. The RARA trinary fusion will result in LBD-H12 or H11_12 castration due to the 3' splicing, then lead to losing responsiveness to ATRA via an allosteric disability mechanism (Fig. 1C, 1D). Experiments confirmed that cells transfected with STAT3::RARA fusion with intact LBD responded well to ATRA, while both the fusion in case #A1 and artificial fusion with LBD-H12 castration showed no response to physiological and pharmacological concentrations of ATRA (Fig. 1E).
Conclusions
This is the first report of pathological trinary fusion, the TEs and transposition mechanism participating in composing pathological fusion genes, and the RARA LBD castration confers ATRA resistance in X::RARA-APL. Also, there might be another synergistic mechanism of RARA 5' fusion and LBD mutation. The formation of trinary fusion and gain of LBD mutation, in addition to X::RARA fusion, requires more genome splicing or transposition or mutation events, which explains the rarity of X::RARA-APL. This study highlights mechanisms of partner gene-associated trinary fusion and the orchestrating of multiple molecular events in the leukemogenesis in X::RARA-APL cases.
Disclosures
No relevant conflicts of interest to declare.